Tethered spinal cord syndrome represents one of the most critical yet often overlooked neurological conditions affecting infants during their earliest developmental stages. This complex disorder occurs when the spinal cord becomes abnormally attached to surrounding tissues within the spinal canal, creating pathological tension that restricts normal cord mobility. Unlike the healthy state where the spinal cord floats freely within cerebrospinal fluid, a tethered cord experiences mechanical stress that intensifies as the child grows, potentially leading to progressive neurological deterioration. Understanding the subtle yet significant early manifestations of this condition is crucial for parents, paediatricians, and healthcare professionals, as prompt recognition can dramatically influence long-term outcomes and prevent irreversible neurological damage.
Neurological manifestations of tethered cord syndrome in neonates
The neurological presentation of tethered cord syndrome in infants often begins with subtle abnormalities that may initially escape clinical detection. Unlike older children who can articulate pain or discomfort, infants rely entirely on observable clinical signs and behavioural changes to communicate their neurological status. The pathophysiology underlying these manifestations involves mechanical traction on neural tissues, leading to compromised blood flow, altered cellular metabolism, and progressive axonal dysfunction within the affected spinal cord segments.
Progressive lower limb weakness and hypotonia patterns
One of the earliest and most concerning neurological manifestations in infants with tethered cord syndrome involves the development of progressive lower limb weakness accompanied by distinctive hypotonia patterns. These infants may initially present with decreased spontaneous leg movements, reduced kicking activity, and an overall diminished muscle tone in the lower extremities. Parents often describe their infant as being “floppy” or having legs that seem less active compared to typical developmental milestones.
The weakness typically follows an ascending pattern , beginning in the distal muscles of the feet and gradually progressing proximally towards the hips and pelvis. This progression reflects the anatomical distribution of nerve roots most susceptible to tethering effects, particularly those originating from the lower lumbar and sacral regions. Clinical examination may reveal decreased muscle bulk in the calves and thighs, reduced resistance to passive movement, and delayed achievement of motor milestones such as leg lifting during diaper changes.
Asymmetric reflexes and babinski sign abnormalities
Reflex abnormalities represent another crucial neurological indicator of tethered cord syndrome in infants. The deep tendon reflexes, particularly the ankle jerks and knee reflexes, may demonstrate asymmetric responses or complete absence on one or both sides. This asymmetry often correlates with the location and severity of spinal cord tethering, providing valuable localising information for healthcare providers.
The Babinski reflex, normally present and expected in healthy infants, may show distinctive abnormalities in cases of tethered cord syndrome. Rather than the typical upward movement of the great toe with fanning of the other toes, affected infants might display absent responses, asymmetric patterns, or paradoxical responses that suggest upper motor neuron dysfunction at the spinal cord level.
Sensory deficits in L4-S2 dermatome distribution
Sensory abnormalities in tethered cord syndrome typically manifest within the L4-S2 dermatome distribution , encompassing the lower legs, feet, perineum, and perianal regions. In infants, these sensory deficits present unique assessment challenges, as traditional sensory testing methods are not applicable to non-verbal patients. However, careful observation may reveal decreased responses to touch, temperature changes, or painful stimuli in the affected dermatomes.
Parents may notice their infant’s reduced reaction to diaper changes, particularly during cleaning of the perineal area, or diminished responses to temperature variations during bathing. Some infants may demonstrate altered pain responses, appearing less distressed during medical procedures involving the lower extremities or perineal region compared to procedures affecting areas with intact sensation.
Neurogenic bladder dysfunction and Detrusor-Sphincter dyssynergia
Neurogenic bladder dysfunction represents one of the most significant early manifestations of tethered cord syndrome, often presenting as detrusor-sphincter dyssynergia or incomplete bladder emptying patterns. In healthy infants, bladder function typically follows predictable patterns with coordinated detrusor muscle contractions and sphincter relaxation. However, infants with tethered cord syndrome may demonstrate disrupted coordination between these essential components of normal voiding.
Clinical indicators may include unusually frequent or infrequent urination, persistent dribbling, or difficulty initiating urination. Some infants may present with recurrent urinary tract infections despite adequate hygiene measures, suggesting incomplete bladder emptying and subsequent bacterial overgrowth. The neurogenic bladder complications can progress rapidly if left untreated, potentially leading to hydronephrosis, vesicoureteral reflux, and chronic kidney dysfunction.
Cutaneous stigmata and external physical markers
The skin overlying the spine often provides the most accessible and visible clues to underlying spinal cord tethering in infants. These cutaneous stigmata serve as external markers of deeper developmental abnormalities and should prompt immediate neurological evaluation. The presence of these skin changes reflects the embryological relationship between neural tube development and overlying ectodermal structures, making them reliable indicators of potential spinal cord anomalies.
Lumbar lipoma and subcutaneous mass formation
Lumbar lipomas represent one of the most common cutaneous manifestations associated with tethered cord syndrome in infants. These subcutaneous fatty masses typically appear as soft, mobile lumps located over the lower lumbar or sacral regions of the spine. Unlike simple subcutaneous lipomas found elsewhere on the body, spinal lipomas often extend deeper into the spinal canal, directly contributing to cord tethering through fibrous adhesions and mechanical compression.
The clinical appearance of these lipomas may vary significantly, ranging from small, barely palpable masses to large, prominent swellings that are readily apparent to parents and healthcare providers. The overlying skin may appear normal or demonstrate subtle changes such as increased warmth, altered texture, or slight discolouration. Careful palpation often reveals the characteristic soft, doughy consistency typical of fatty tissue, though deeper components may be difficult to assess through physical examination alone.
Dermal sinus tract and dimple characteristics
Dermal sinus tracts and associated dimples represent another critical category of cutaneous stigmata that may indicate underlying tethered cord syndrome. These epithelium-lined tracts can extend from the skin surface deep into the spinal canal, potentially creating direct communication between the external environment and the central nervous system. The clinical significance of these findings cannot be overstated, as they may predispose infants to serious complications including meningitis and spinal abscess formation.
The appearance of dermal sinus tracts varies considerably, from simple shallow dimples to deep, narrow openings that may discharge clear or purulent material. The location of these findings is particularly important, with dimples located above the upper border of the gluteal cleft being more likely to represent true dermal sinus tracts requiring immediate medical attention. Healthcare providers must carefully distinguish between benign sacral dimples, which are common and typically harmless, and pathological dermal sinus tracts that may indicate serious underlying abnormalities.
Hypertrichosis and capillary haemangioma presentation
Localised hypertrichosis , characterised by excessive hair growth over the lower spine, represents another important cutaneous marker of potential tethered cord syndrome. This abnormal hair growth typically appears as a tuft or patch of dark, coarse hair located over the lumbar or sacral regions, contrasting sharply with the fine, sparse hair normally present in infants. The hypertrichosis may be present at birth or develop during the first few months of life as the infant’s hair patterns become more established.
Capillary haemangiomas, appearing as flat or slightly raised reddish-purple skin lesions, may also indicate underlying spinal abnormalities. These vascular malformations often have irregular borders and may demonstrate changes in colour or size over time. The combination of hypertrichosis and capillary haemangiomas over the spinal region should raise particular concern for underlying neural tube defects and associated tethered cord syndrome.
Asymmetric gluteal cleft and sacral agenesis signs
Careful examination of the gluteal region may reveal asymmetric gluteal cleft patterns that suggest underlying sacral abnormalities associated with tethered cord syndrome. A normal gluteal cleft typically appears as a straight, symmetrical furrow extending from the lower back towards the anal region. However, infants with spinal abnormalities may demonstrate clefts that deviate to one side, appear forked, or terminate prematurely.
Signs of sacral agenesis, including flattened buttocks, decreased gluteal muscle bulk, or altered anal positioning, may accompany tethered cord syndrome. These findings reflect the close embryological relationship between sacral vertebral development and the terminal portions of the spinal cord, making them important clinical indicators of potential neurological involvement.
Orthopaedic complications and musculoskeletal deformities
The orthopaedic manifestations of tethered cord syndrome in infants represent a complex interplay between neurological dysfunction and progressive musculoskeletal deformity. As the condition progresses, the combination of muscle weakness, altered innervation patterns, and growth-related changes can lead to significant structural abnormalities that may become increasingly difficult to correct with time. Understanding these early orthopaedic changes is crucial for healthcare providers, as prompt intervention can prevent the development of severe deformities and preserve functional mobility.
One of the most characteristic early orthopaedic findings involves the development of foot deformities , particularly those affecting the shape and positioning of the feet and ankles. These deformities often begin subtly, with parents noticing that one foot appears different from the other or that their infant’s feet seem to assume unusual positions during rest or activity. The most common presentations include clubfoot deformities, characterised by inward turning and downward pointing of the affected foot, and cavovarus deformities, which involve high arches combined with inward tilting of the heel.
Progressive scoliosis represents another significant concern in infants with tethered cord syndrome, though it may not become apparent until later in infancy or early childhood. The spinal curvature typically develops gradually, beginning as a mild asymmetry that may initially be attributed to positioning preferences or muscle imbalances. However, unlike functional scoliosis caused by external factors, the spinal curvature associated with tethered cord syndrome tends to be progressive and may not respond to conservative management approaches such as positioning or physical therapy alone.
Hip dysplasia and associated abnormalities in lower limb alignment may also complicate the clinical picture in infants with tethered cord syndrome. The altered muscle tone and innervation patterns can affect the normal development of the hip joint, potentially leading to shallow acetabuli, subluxation, or frank dislocation. These changes may be subtle in early infancy but can progress rapidly during periods of growth, emphasising the importance of regular orthopaedic surveillance in at-risk infants.
The early recognition of orthopaedic abnormalities in tethered cord syndrome can significantly impact long-term functional outcomes, as many deformities are more amenable to correction when addressed promptly.
Urological symptoms and genitourinary dysfunction patterns
The urological manifestations of tethered cord syndrome in infants represent some of the most clinically significant and potentially devastating complications of this condition. The neural pathways controlling bladder and bowel function are particularly vulnerable to the effects of spinal cord tethering, as they involve complex coordination between multiple nerve roots and spinal cord segments. Early recognition of urological dysfunction is paramount, as delayed diagnosis and treatment can result in irreversible kidney damage and lifelong complications.
Neurogenic bladder with incomplete emptying
Neurogenic bladder dysfunction in infants with tethered cord syndrome typically manifests as incomplete bladder emptying combined with altered voiding patterns. Unlike healthy infants who demonstrate coordinated detrusor contractions with sphincter relaxation, affected infants may show dyssynergic patterns where the bladder muscle and urethral sphincter work against each other rather than in harmony. This dysfunction can be particularly challenging to detect in infants, as normal voiding patterns vary considerably during the first year of life.
Parents may notice several subtle signs suggesting neurogenic bladder dysfunction, including unusually wet diapers that seem disproportionately heavy for the infant’s fluid intake, constant dribbling of urine rather than discrete voiding episodes, or conversely, periods where diapers remain surprisingly dry followed by sudden large-volume urination. Some infants may demonstrate straining behaviours during attempted urination, appearing uncomfortable or distressed without obvious cause.
Vesicoureteral reflux and hydronephrosis development
The combination of incomplete bladder emptying and elevated intravesical pressures associated with neurogenic bladder can lead to the development of vesicoureteral reflux , a condition where urine flows backward from the bladder into the ureters and potentially into the kidneys. This retrograde flow of urine creates ideal conditions for bacterial growth and can result in recurrent urinary tract infections, which may be among the first clinical signs prompting medical evaluation in affected infants.
Progressive hydronephrosis, characterised by dilatation of the renal collecting system, represents a more advanced complication that can develop insidiously in infants with untreated neurogenic bladder. The condition may be asymptomatic initially, making routine urological surveillance essential for early detection. When hydronephrosis does progress to symptomatic levels, infants may present with failure to thrive, feeding difficulties, irritability, or recurrent fever episodes associated with urinary tract infections.
Sphincter dysfunction and incontinence mechanisms
Sphincter dysfunction in tethered cord syndrome involves complex abnormalities in both the internal and external urethral sphincters, leading to distinctive patterns of incontinence mechanisms that differ from those seen in other urological conditions. The internal sphincter, composed of smooth muscle and controlled by sympathetic innervation, may demonstrate reduced tone or inappropriate relaxation, while the external sphincter, consisting of striated muscle under voluntary control, may show paradoxical contractions or failure to relax appropriately during voiding attempts.
These sphincter abnormalities create characteristic clinical patterns that experienced healthcare providers can recognise through careful observation and urodynamic testing when indicated. Infants may demonstrate continuous low-grade urinary leakage combined with periodic episodes of complete urinary retention, or they may show apparently normal voiding patterns that mask underlying high-pressure bladder dysfunction detectable only through specialised testing procedures.
Diagnostic imaging findings and MRI conus medullaris assessment
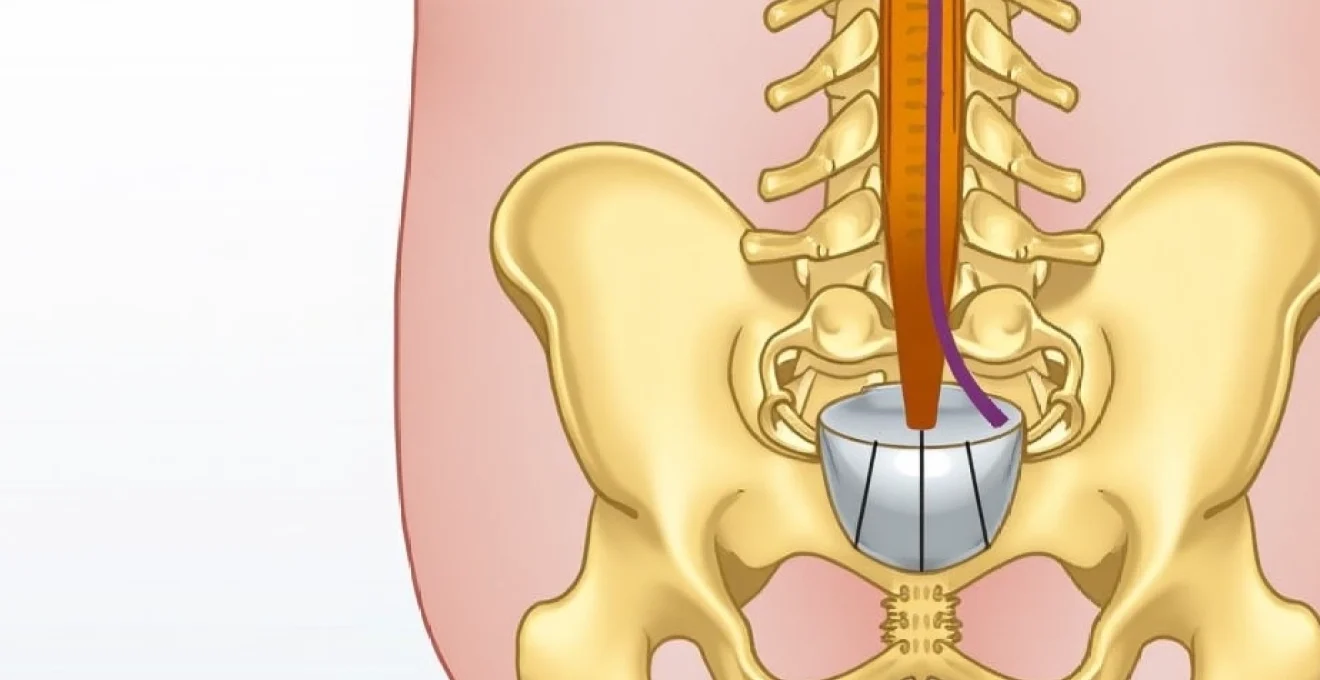
Advanced neuroimaging techniques, particularly magnetic resonance imaging (MRI), play an indispensable role in confirming the diagnosis of tethered cord syndrome and guiding subsequent management decisions. The conus medullaris assessment represents the cornerstone of radiological evaluation, as it provides detailed information about spinal cord position, morphology, and associated anatomical abnormalities that may not be apparent through clinical examination alone. Understanding the nuanced imaging findings associated with tethered cord syndrome is essential for healthcare providers, as these studies often determine the timing and approach to surgical intervention.
The normal position of the conus medullaris varies with age, typically residing at the L2-L3 level in newborns and gradually ascending to the L1-L2 level by two years of age. In cases of tethered cord syndrome, the conus may be positioned abnormally low, often below the L2-L3 level in infants, though it’s important to note that conus position alone is not diagnostic, as some healthy infants may have a low-lying conus without clinical significance. The key imaging findings that support a diagnosis of tethered cord syndrome include the identification of tethering lesions such as lipomas, fibrous bands, or scar tissue that restrict normal cord mobility.
Specialised MRI sequences, including T1-weighted and T2-weighted images in multiple planes, provide detailed visualisation of spinal cord anatomy and surrounding structures. The sagittal images are particularly valuable for assessing conus position and identifying longitudinal abnormalities, while axial images help characterise the relationship between the spinal cord and potential tethering elements. Dynamic MRI studies, though not
routinely performed in all centres, can provide additional information about cord mobility and tethering severity by demonstrating restricted movement during flexion and extension manoeuvres.
Additional imaging findings that may accompany tethered cord syndrome include syringomyelia, characterised by fluid-filled cavities within the spinal cord parenchyma, and associated vertebral anomalies such as hemivertebrae, butterfly vertebrae, or segmentation abnormalities. These findings help establish the underlying developmental nature of the condition and may influence surgical planning approaches. The presence of a thickened filum terminale, appearing as a prominent fibrous band extending from the conus medullaris to the sacrum, represents another important imaging marker that can be readily identified on high-resolution MRI studies.
Ultrasonography may serve as a valuable complementary imaging modality in very young infants, particularly those under three months of age when the posterior elements of the spine remain incompletely ossified. This technique allows real-time assessment of spinal cord mobility and can help identify obvious tethering elements without requiring sedation. However, ultrasound has significant limitations in detecting subtle abnormalities and cannot provide the detailed anatomical information necessary for surgical planning, making MRI the gold standard for comprehensive evaluation of suspected tethered cord syndrome.
Early intervention strategies and neurosurgical management protocols
The management of tethered cord syndrome in infants requires a delicate balance between the need for timely intervention and the inherent risks associated with neurosurgical procedures in this vulnerable population. Early intervention strategies focus on preventing progressive neurological deterioration while maximising the potential for functional recovery and normal development. The timing of surgical intervention represents one of the most critical decisions in the management of these infants, as delayed treatment may result in irreversible neurological damage, while premature intervention carries significant procedural risks.
The primary neurosurgical approach involves detethering procedures designed to release the spinal cord from abnormal attachments and restore normal cord mobility within the spinal canal. These procedures typically require microsurgical techniques performed under general anaesthesia, with careful intraoperative monitoring to minimise the risk of neurological injury. The specific surgical approach varies depending on the underlying cause of tethering, with simple cases involving thickened filum terminale requiring relatively straightforward division, while complex cases associated with lipomas or extensive scar tissue may necessitate more extensive dissection and reconstruction.
Intraoperative neurophysiological monitoring plays a crucial role in modern neurosurgical management protocols, allowing real-time assessment of spinal cord function during the procedure. These monitoring techniques include somatosensory evoked potentials, motor evoked potentials, and electromyographic recordings from relevant muscle groups, providing immediate feedback about the integrity of neural pathways. The use of such monitoring has significantly improved surgical outcomes and reduced the incidence of iatrogenic neurological complications in infants undergoing detethering procedures.
The surgical technique typically involves a posterior midline approach with careful dissection through the subcutaneous tissues and paraspinal muscles to expose the affected spinal levels. Microscopic visualisation is essential for identifying the precise location and extent of tethering elements, allowing surgeons to perform targeted release while preserving normal neural structures. In cases involving lipomatous tissue, complete removal may not be possible or advisable, and the primary goal shifts to achieving adequate cord mobilisation while minimising surgical morbidity.
Postoperative management protocols emphasise careful neurological monitoring and early mobilisation when appropriate. Infants typically require intensive care unit monitoring for the first 24-48 hours following surgery, with particular attention to neurological status, wound healing, and potential complications such as cerebrospinal fluid leakage. The development of standardised postoperative care pathways has helped optimise outcomes and reduce length of stay for these complex patients.
Long-term follow-up represents an essential component of comprehensive management protocols, as infants with tethered cord syndrome require ongoing surveillance for potential retethering, particularly during periods of rapid growth. Regular neurological examinations, developmental assessments, and interval imaging studies help detect early signs of recurrent tethering, allowing for prompt intervention when necessary. Studies suggest that approximately 10-20% of infants may require repeat surgery due to retethering, emphasising the importance of lifelong medical surveillance.
The success of early intervention in tethered cord syndrome depends not only on surgical expertise but also on comprehensive multidisciplinary care that addresses the complex medical needs of these vulnerable infants throughout their development.
Multidisciplinary care coordination involves collaboration between neurosurgeons, paediatric neurologists, urologists, orthopaedic surgeons, and rehabilitation specialists to address the various complications associated with tethered cord syndrome. This team-based approach ensures that all aspects of the infant’s care are optimised, from immediate surgical management to long-term developmental support and complication prevention. The integration of family education and support services also plays a vital role in achieving optimal outcomes, as parents must understand the signs and symptoms that warrant immediate medical attention.
Recent advances in surgical techniques, including the use of laser technology and improved imaging guidance systems, have further enhanced the safety and efficacy of detethering procedures in infants. These technological improvements allow for more precise tissue dissection, reduced thermal damage to surrounding structures, and improved visualisation of critical anatomical landmarks. The continued evolution of these techniques promises even better outcomes for infants diagnosed with tethered cord syndrome in the future.
Prevention strategies, while limited, focus primarily on optimising maternal health during pregnancy through adequate folic acid supplementation and avoiding known teratogenic exposures. Public health initiatives promoting preconceptional folic acid supplementation have demonstrated significant reductions in neural tube defects, which represent the primary risk factor for developing tethered cord syndrome. Early prenatal diagnosis through advanced imaging techniques also allows for improved perinatal planning and preparation for postnatal management needs.
The prognosis for infants with tethered cord syndrome varies considerably depending on the severity of the condition, the timing of diagnosis and intervention, and the presence of associated abnormalities. Those diagnosed and treated early, particularly before the development of significant neurological deficits, generally have excellent outcomes with normal or near-normal neurological function. However, infants with delayed diagnosis or complex underlying abnormalities may experience persistent deficits despite optimal surgical management, highlighting the critical importance of early recognition and prompt treatment of this challenging condition.