Scalp numbness, medically termed scalp paresthesia, represents a complex neurological symptom characterised by altered sensation across the cranial surface. This condition manifests as diminished feeling, tingling sensations, or complete loss of tactile perception in specific areas of the head. The phenomenon affects approximately 2-5% of the adult population at some point during their lifetime, with higher prevalence rates observed amongst individuals over 50 years of age.
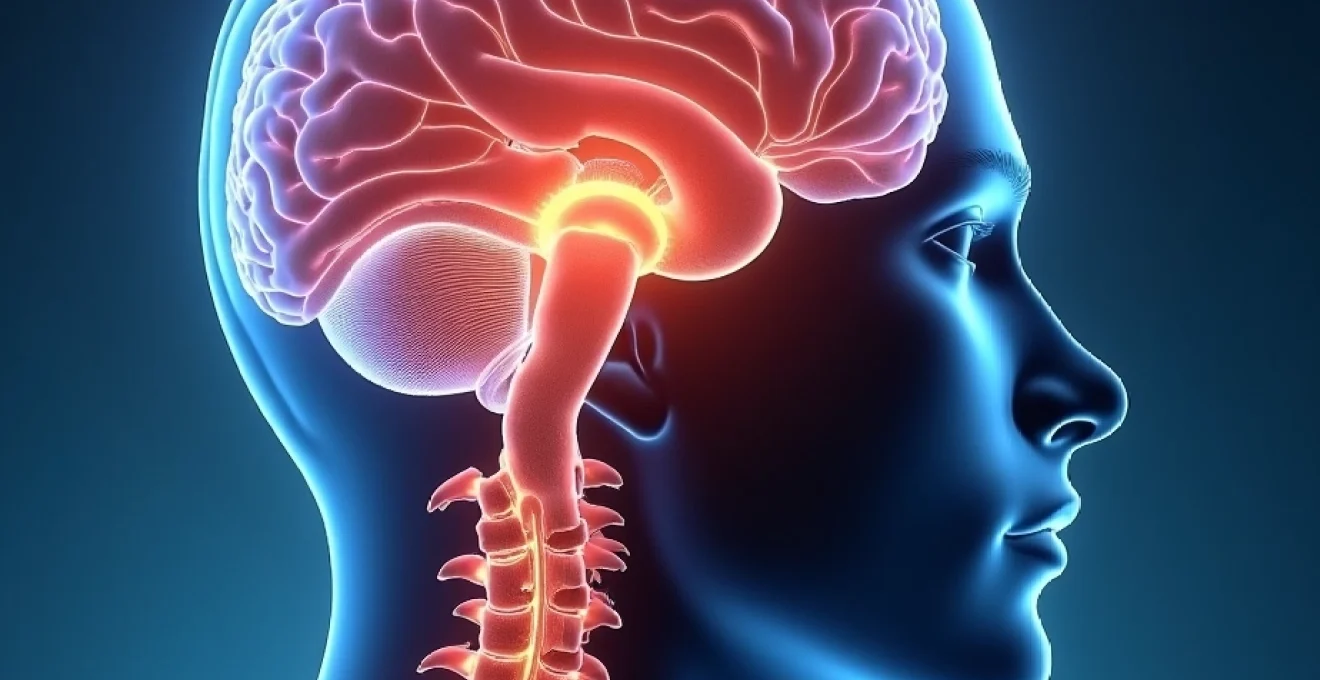
Understanding the underlying mechanisms behind scalp numbness requires examining the intricate network of sensory pathways that innervate the cranial region. The scalp receives sensory input through multiple nerve distributions , including branches of the trigeminal nerve, occipital nerves, and upper cervical nerve roots. When these pathways experience disruption, whether through neurological disease, vascular compromise, or mechanical compression, patients may develop various degrees of sensory dysfunction.
The diagnostic complexity of scalp numbness stems from its diverse aetiology, ranging from benign tension headaches to serious neurological conditions requiring immediate medical intervention. Recent epidemiological studies indicate that approximately 60% of scalp numbness cases result from neurological disorders, whilst 25% originate from vascular insufficiency and 15% from mechanical compression syndromes.
Neurological conditions affecting trigeminal and occipital nerve pathways
The nervous system’s role in scalp sensation involves complex interactions between central and peripheral pathways. Neurological conditions affecting these systems frequently present with scalp numbness as an early or prominent symptom. Understanding these conditions requires examining both the anatomical distribution of cranial nerves and the pathophysiological processes that disrupt normal sensory transmission.
Multiple sclerosis demyelination in cranial nerve distribution
Multiple sclerosis (MS) affects approximately 130,000 individuals in the United Kingdom, with cranial nerve involvement occurring in roughly 40% of patients during the disease course. The demyelinating process characteristic of MS can affect any portion of the trigeminal nerve complex, leading to distinctive patterns of scalp numbness. Patients typically describe unilateral facial and scalp sensory disturbances that may progress over days to weeks.
The pathophysiology involves inflammatory destruction of myelin sheaths surrounding cranial nerve fibres, resulting in slowed or blocked nerve conduction. Trigeminal nerve demyelination in MS patients often presents with burning sensations alongside numbness, particularly affecting the ophthalmic division which innervates the forehead and anterior scalp regions. Magnetic resonance imaging studies reveal characteristic demyelinating plaques in the brainstem tegmentum and trigeminal nerve root entry zones in approximately 75% of affected patients.
Trigeminal neuralgia and atypical facial pain syndromes
Classical trigeminal neuralgia affects roughly 4-5 individuals per 100,000 annually, predominantly occurring in patients over 50 years of age. Whilst typically characterised by severe shooting pains, atypical presentations may include profound numbness across trigeminal nerve distributions. The condition results from vascular compression of the trigeminal nerve root, most commonly by the superior cerebellar artery or anterior inferior cerebellar artery.
Atypical facial pain syndromes present differently, often involving constant burning or aching sensations accompanied by areas of scalp numbness. These conditions frequently affect the second and third divisions of the trigeminal nerve, causing numbness extending from the temple region across the lateral scalp. Patients with secondary trigeminal neuralgia due to multiple sclerosis or tumours may experience more extensive sensory loss compared to classical presentations.
Occipital neuralgia from C2-C3 nerve root compression
Occipital neuralgia represents a distinct clinical entity characterised by sharp, shooting pains originating from the suboccipital region and radiating over the posterior scalp. The condition affects the greater and lesser occipital nerves, which arise from the C2 and C3 spinal nerve roots respectively. Compression or irritation of these nerve roots can produce significant numbness throughout their sensory distribution.
The greater occipital nerve provides sensation to approximately two-thirds of the posterior scalp, whilst the lesser occipital nerve innervates the lateral aspects behind the ears. Cervical spine pathology at the C1-C2 or C2-C3 levels frequently underlies occipital neuralgia , with arthritis, disc degeneration, or atlantooccipital joint dysfunction serving as common precipitating factors. Patients typically experience numbness following episodes of severe pain, creating a characteristic pattern of alternating hyperalgesia and hypoesthesia.
Peripheral neuropathy in diabetes mellitus type 2
Diabetic neuropathy affects approximately 50% of individuals with long-standing diabetes mellitus, with cranial nerve involvement occurring in 1-2% of diabetic patients. Small fibre neuropathy associated with diabetes can affect the terminal branches of cranial nerves supplying scalp sensation, resulting in patchy areas of numbness or altered sensation. The pathophysiology involves chronic hyperglycaemia-induced damage to nerve fibres and supporting vasculature.
Diabetic cranial neuropathies typically develop insidiously over months to years, contrasting with the acute onset characteristic of diabetic cranial nerve palsies. Patients frequently report burning sensations progressing to numbness across the forehead and scalp regions, particularly in areas supplied by the supraorbital and supratrochlear nerves. Glycaemic control plays a crucial role in symptom progression, with HbA1c levels above 7% associated with increased neuropathy severity.
Post-herpetic neuralgia following herpes zoster outbreaks
Herpes zoster affecting cranial dermatomes occurs in approximately 10-15% of all shingles cases, with the trigeminal nerve representing the most commonly affected cranial nerve. Post-herpetic neuralgia develops in roughly 20% of patients following cranial herpes zoster, creating persistent sensory disturbances that may include profound numbness alongside neuropathic pain.
The viral reactivation process damages both sensory nerve fibres and dorsal root ganglia, leading to altered pain processing and sensory transmission. Scalp numbness following herpes zoster ophthalmicus affects the distribution of the ophthalmic division of the trigeminal nerve, encompassing the forehead, upper eyelid, and anterior scalp regions. Age represents the most significant risk factor, with patients over 60 years experiencing post-herpetic neuralgia rates exceeding 40%.
Vascular insufficiency and cerebrovascular disorders
Cerebrovascular conditions affecting scalp sensation involve complex interactions between arterial supply, venous drainage, and neural tissue perfusion. The scalp’s rich vascular network, supplied primarily by branches of the external carotid artery, maintains the metabolic demands of sensory nerve endings and supporting structures. When vascular compromise occurs, either through arterial insufficiency or venous congestion, patients may develop various patterns of scalp numbness.
Vascular causes of scalp numbness often present with accompanying symptoms such as headaches, visual disturbances, or cognitive changes, reflecting the interconnected nature of cerebral and scalp circulation.
Vertebrobasilar insufficiency syndrome
Vertebrobasilar insufficiency affects the posterior circulation of the brain, including areas responsible for processing cranial nerve sensory input. This condition typically results from atherosclerotic narrowing of the vertebral or basilar arteries, leading to intermittent or chronic hypoperfusion of brainstem structures. Patients may experience scalp numbness as part of a broader constellation of posterior circulation symptoms.
The trigeminal sensory nucleus, located within the brainstem, receives diminished blood flow during vertebrobasilar insufficiency episodes. Patients frequently report numbness affecting multiple cranial nerve distributions simultaneously , often accompanied by dizziness, diplopia, or ataxia. Transcranial Doppler studies reveal reduced flow velocities in the posterior circulation during symptomatic episodes, with mean flow velocities typically below 30 cm/second indicating significant stenosis.
Transient ischaemic attacks in posterior circulation
Transient ischaemic attacks (TIAs) affecting the posterior circulation can produce temporary scalp numbness through involvement of brainstem sensory processing centres. These episodes typically last minutes to hours and may recur over days to weeks. The posterior inferior cerebellar artery territory, when affected, can influence trigeminal sensory processing through brainstem hypoperfusion.
Posterior circulation TIAs present unique diagnostic challenges due to their varied symptom patterns and tendency for symptom fluctuation. Scalp numbness during these episodes may affect unilateral or bilateral distributions , depending on the specific vascular territory involved. Diffusion-weighted MRI sequences reveal acute ischaemic changes in approximately 30% of patients presenting with posterior circulation TIA symptoms within 24 hours of symptom onset.
Temporal arteritis and giant cell arteritis
Giant cell arteritis (GCA) represents a systemic vasculitis primarily affecting large and medium-sized arteries in individuals over 50 years of age. The condition has a predilection for cranial arteries, including the superficial temporal artery, occipital artery, and posterior auricular artery. Inflammation of these vessels can compromise scalp perfusion and affect sensory nerve function.
The inflammatory process characteristic of GCA involves granulomatous infiltration of arterial walls, leading to vessel wall thickening and luminal narrowing. Scalp numbness in GCA patients often accompanies scalp tenderness and temporal headaches , creating a distinctive clinical pattern. Temporal artery biopsy remains the diagnostic gold standard, with positive findings present in approximately 85% of patients when performed within 14 days of symptom onset.
Cervical artery dissection complications
Cervical artery dissections, whilst uncommon, can produce scalp numbness through several mechanisms. Vertebral artery dissections may compromise posterior circulation blood flow, affecting brainstem centres responsible for cranial nerve sensory processing. Internal carotid artery dissections can influence trigeminal nerve function through altered perfusion of nerve root entry zones.
The pathophysiology involves intimal tears within cervical arteries, leading to intramural haematoma formation and subsequent luminal compromise. Patients with cervical artery dissection may experience scalp numbness alongside neck pain, headache, and neurological deficits . CT angiography and MR angiography demonstrate dissection features in over 95% of cases, with characteristic findings including arterial narrowing, intramural haematoma, and pseudoaneurysm formation.
Mechanical compression and cervical spine pathology
Mechanical compression syndromes affecting scalp sensation involve anatomical structures that can impinge upon sensory nerve pathways. The cervical spine’s complex anatomy, including vertebral bodies, facet joints, intervertebral discs, and surrounding musculature, creates multiple potential sites for nerve compression. Understanding these mechanical factors requires examining both static anatomical relationships and dynamic movement patterns that may exacerbate compression.
Cervical spondylosis with neural foraminal stenosis
Cervical spondylosis represents a degenerative process affecting the cervical spine, with neural foraminal stenosis occurring in approximately 25% of individuals over 50 years of age. When stenosis affects the C2-C3 or C3-C4 levels, compression of nerve roots contributing to scalp innervation can produce numbness patterns corresponding to specific dermatomes.
The degenerative process involves disc height loss, osteophyte formation, and ligamentum flavum hypertrophy, collectively reducing neural foraminal dimensions. Patients with cervical spondylosis may experience scalp numbness that worsens with neck extension or rotation , reflecting positional compression of affected nerve roots. Advanced imaging with MRI demonstrates neural foraminal narrowing in over 80% of symptomatic patients, with foraminal cross-sectional areas reduced by 30-50% compared to normal values.
Atlantoaxial subluxation and upper cervical instability
Atlantoaxial subluxation involves abnormal movement between the C1 and C2 vertebrae, potentially affecting the C2 nerve root which contributes significantly to posterior scalp innervation through the greater occipital nerve. This condition may result from trauma, inflammatory arthritis, or congenital abnormalities affecting upper cervical spine stability.
The C2 nerve root passes through a confined space between the posterior arch of C1 and the superior articular process of C2. Atlantoaxial instability can create intermittent compression of this nerve root , producing episodic scalp numbness that may correlate with specific head positions or movements. Dynamic cervical spine imaging reveals abnormal atlantoaxial motion patterns, with atlantodental intervals exceeding 3mm in adults indicating clinically significant instability.
Tension-type headache with myofascial trigger points
Chronic tension-type headaches frequently involve myofascial dysfunction affecting muscles of the scalp, neck, and shoulders. Trigger points within the occipitalis, frontalis, temporalis, and upper trapezius muscles can create referred pain patterns and sensory disturbances extending across the scalp. These trigger points represent hyperirritable spots within muscle tissue that can affect local circulation and nerve function.
The pathophysiology involves sustained muscle contraction and metabolic dysfunction within affected muscle fibres, leading to local hypoxia and accumulation of inflammatory mediators. Myofascial trigger points can compress small sensory nerve branches as they traverse muscle tissue, producing areas of numbness or altered sensation. Manual examination reveals tender nodules within affected muscles, with patients reporting reproduction of familiar symptoms during trigger point palpation.
Whiplash-associated disorders grade II-III
Whiplash-associated disorders (WAD) encompass a spectrum of injuries resulting from sudden acceleration-deceleration forces affecting the cervical spine. Grade II-III whiplash injuries involve significant soft tissue damage and may include facet joint capsule tears, muscle strains, and nerve root irritation. These injuries can produce persistent scalp numbness through multiple mechanisms.
The biomechanics of whiplash injuries create complex patterns of tissue damage, including stretching or compression of upper cervical nerve roots and their peripheral branches. Patients with moderate to severe whiplash injuries may develop scalp numbness weeks to months after the initial trauma , reflecting ongoing inflammatory processes and scar tissue formation affecting nerve structures. Quantitative sensory testing reveals altered sensation thresholds in over 70% of patients with chronic whiplash-associated disorders.
Dermatological and Scalp-Specific conditions
Dermatological conditions affecting the scalp can produce numbness through various mechanisms, including direct nerve involvement, inflammatory processes, and structural changes within skin and subcutaneous tissues. The scalp’s unique anatomy, characterised by dense hair follicles, sebaceous glands, and rich innervation, creates specific patterns of disease expression that may differ from other body regions.
Scalp dysesthesia represents a particularly relevant condition characterised by abnormal sensations, including numbness, burning, or tingling, occurring without visible skin changes. This neurogenic condition affects approximately 1-3% of dermatology patients, with higher prevalence rates observed in post-menopausal women and individuals with diabetes mellitus. The pathophysiology involves dysfunction of small-diameter sensory nerve fibres within the scalp, leading to altered pain and touch sensation processing.
Seborrheic dermatitis affects the scalp in approximately 5% of the adult population, creating inflammatory changes that can influence local nerve function. The condition involves overgrowth of Malassezia species fungi, leading to inflammatory responses that may affect sensory nerve endings. Patients frequently report altered scalp sensation, including areas of numbness alternating with hyperesthesia, particularly during active disease phases.
Alopecia areata, an autoimmune condition affecting hair follicles, can produce scalp numbness through inflammatory processes affecting perifollicular nerve networks. The condition affects approximately 2% of the population at some point during their lifetime, with scalp sensory changes reported in roughly 20% of patients during active phases. The inflammatory infiltrate characteristic of alopecia areata can exten
d beyond hair follicles to affect surrounding nerve structures, creating zones of altered sensation that may persist even after hair regrowth occurs.Scalp psoriasis affects approximately 50% of individuals diagnosed with psoriasis elsewhere on the body, creating thick, scaly plaques that can mechanically compress superficial nerve endings. The inflammatory cytokines associated with psoriatic lesions, particularly tumor necrosis factor-alpha and interleukin-17, can directly affect nerve function and contribute to sensory disturbances. Patients with extensive scalp psoriasis frequently report numbness alternating with intense itching, reflecting the complex interaction between inflammation and nerve dysfunction.Folliculitis decalvans, a rare cicatricial alopecia, involves chronic inflammatory destruction of hair follicles with subsequent scarring. The scarring process can entrap or damage small sensory nerve branches, leading to permanent areas of scalp numbness. This condition affects fewer than 1% of patients with hair loss disorders but demonstrates how inflammatory scalp conditions can produce lasting sensory changes through structural tissue alterations.
Pharmaceutical and toxicological causes
Pharmaceutical agents represent a significant but often overlooked cause of scalp numbness, affecting an estimated 8-12% of patients experiencing medication-induced peripheral neuropathy. The mechanisms through which medications produce scalp numbness vary considerably, ranging from direct neurotoxic effects to metabolic disruption of nerve function. Understanding these drug-induced causes becomes increasingly important as polypharmacy rates continue rising, particularly among elderly populations.
Chemotherapeutic agents, particularly platinum-based compounds such as cisplatin and carboplatin, demonstrate well-documented neurotoxic properties affecting both peripheral and cranial nerves. These agents accumulate within dorsal root ganglia and cranial nerve ganglia, disrupting cellular metabolism and leading to axonal degeneration. Patients receiving platinum-based chemotherapy develop scalp numbness in approximately 30-40% of cases, with symptoms typically emerging after the second or third treatment cycle.
Anticonvulsant medications, including phenytoin, carbamazepine, and newer agents such as gabapentin, can paradoxically produce sensory disturbances despite their primary indication for neuropathic pain management. The mechanism involves chronic sodium channel blockade, which can impair normal nerve conduction when therapeutic levels are exceeded or in susceptible individuals. Scalp numbness associated with anticonvulsant use typically develops gradually over weeks to months and may be dose-dependent.
Antimicrobial agents present another significant category of medications capable of inducing scalp numbness. Metronidazole, when used for extended periods or at high doses, can produce peripheral neuropathy affecting cranial nerves. The neurotoxic effects result from mitochondrial dysfunction within nerve cells, leading to energy metabolism disruption. Patients receiving prolonged metronidazole therapy develop sensory symptoms in 10-15% of cases, with cranial nerve involvement typically occurring after several weeks of treatment.
Statins, widely prescribed for cardiovascular disease prevention, occasionally produce peripheral neuropathy as an adverse effect. While peripheral neuropathy affects fewer than 1% of statin users, cranial nerve involvement can occur, particularly with higher doses or in patients with pre-existing risk factors such as diabetes mellitus. The mechanism involves disruption of cholesterol biosynthesis within nerve cells, affecting membrane integrity and signal transmission.
Heavy metal toxicity, particularly from lead, mercury, or arsenic exposure, can produce distinctive patterns of cranial nerve dysfunction including scalp numbness. Lead toxicity affects nerve conduction through interference with calcium channels and disruption of neurotransmitter release. Occupational or environmental exposure histories become crucial in identifying these cases, as symptoms may develop insidiously over months to years. Chronic heavy metal exposure can produce permanent sensory changes requiring chelation therapy and removal from the exposure source.
Autoimmune and systemic inflammatory disorders
Autoimmune conditions affecting scalp sensation involve complex interactions between immune system dysfunction and neural tissue damage. These disorders can produce scalp numbness through direct autoantibody-mediated nerve damage, inflammatory infiltration of neural structures, or systemic effects on nerve metabolism and function. The prevalence of autoimmune-related scalp numbness has increased in recognition over recent decades as diagnostic techniques have improved.
Systemic lupus erythematosus (SLE) affects the nervous system in approximately 60% of patients, with cranial nerve involvement occurring in 10-15% of cases. The pathophysiology involves immune complex deposition within nerve vasculature, leading to inflammatory damage and subsequent sensory dysfunction. Patients with SLE may develop scalp numbness as part of broader neuropsychiatric manifestations, often accompanied by cognitive changes, mood disturbances, or other cranial nerve deficits.
Sjögren’s syndrome, primarily recognised for its effects on salivary and lacrimal glands, produces peripheral neuropathy in approximately 20% of patients. The condition involves lymphocytic infiltration of neural tissues, particularly affecting small-diameter sensory fibres. Scalp numbness in Sjögren’s syndrome patients often presents alongside facial numbness and may precede other systemic manifestations by months to years. Anti-SSA/Ro and anti-SSB/La antibodies correlate with increased neuropathy risk.
Sarcoidosis, a multi-system granulomatous disorder, involves the nervous system in approximately 5% of patients, with cranial nerve involvement representing the most common neurological manifestation. Granulomatous infiltration can affect any portion of cranial nerve pathways, from peripheral nerve branches to central processing centres. Scalp numbness may result from direct granulomatous involvement of sensory nerve branches or secondary effects from brainstem infiltration.
Antiphospholipid syndrome creates a hypercoagulable state that can affect cerebral circulation and produce scalp numbness through ischaemic mechanisms. The condition involves circulating antibodies against phospholipid-binding proteins, leading to increased thrombosis risk. Patients with antiphospholipid syndrome may experience fluctuating scalp numbness corresponding to episodes of cerebral hypoperfusion or small vessel thrombosis.
Vasculitis syndromes affecting small to medium-sized vessels can produce scalp numbness through ischaemic damage to sensory nerve structures. Primary central nervous system vasculitis, whilst rare, can affect intracranial portions of cranial nerves. Secondary vasculitis associated with conditions such as rheumatoid arthritis or polyarteritis nodosa may involve scalp vessels and their associated nerve structures, creating patchy areas of sensory loss.
Thyroid disorders, particularly hypothyroidism, can contribute to scalp numbness through effects on peripheral nerve metabolism. Thyroid hormones play crucial roles in nerve conduction velocity and myelin maintenance. Severe hypothyroidism can produce peripheral neuropathy in up to 30% of patients, with cranial nerve involvement occurring less frequently but representing a recognised complication of prolonged hormone deficiency. The mechanism involves reduced protein synthesis and energy metabolism within nerve cells, leading to gradual deterioration of nerve function that typically reverses with appropriate hormone replacement therapy.